Généralités
- All solids, natural or artificial, that are present around us, are at the origin of a well-arranged elementary microscopic component called a crystal.


Preface All solids, whether natural or artificial, that surround us originate from a well-arranged microscopic component called a crystal. The growth, synthesis, and maintenance of crystalline matter are intrinsically linked to the atoms that compose it. Their orderly arrangement results from the laws governing their stability, as well as the physical and chemical properties that distinguish crystals from one another. The science that deals with everything related to crystals is called crystallography. Numerous fields are interested in the study of crystals, including inorganic and organic chemistry, physics, geology, metallurgy, biology, and medicine. This highlights the importance of studying crystals in our daily lives, as nearly all our products and raw materials are crystalline. For example, rocks are composed of minerals, while minerals are formed from an assembly of crystals, which are made up of atoms (motifs) organized into geometric systems called lattice networks.
Mineralogy, the science that studies minerals, relies heavily on crystallography, as certain properties of minerals are linked to the formation of crystals in their deposition environment. Students are expected to understand all crystal systems, their geometry, symmetry elements, and the various modes and classes to which they belong. They must also grasp the fundamental concepts of crystallography, which are taught in the first year of geology.
The first part of the course, titled Geometric Crystallography, focuses on explaining the fundamental concepts of crystallography, such as laws, crystal structures, and their components (lattice and motif), as well as the different bonds formed. It also interprets the notion of symmetry, classes, and associated operations and describes certain properties related to the arrangement of atoms and molecules within a three-dimensional unit cell. The second part, titled Crystal Optics, interprets the instrumentation used for observing and measuring crystals, such as X-ray diffraction and emphasizes understanding the interaction of light with crystalline bodies.
Objectives Understand Crystal Systems, where students should be able to identify and describe all crystal systems, their geometry, and their symmetry elements. Also, students should interpret symmetry operations, crystal classes, and their applications in crystallography
Master Fundamental Concepts, so students must grasp the basic principles of crystallography, including lattice structures, motifs, and atomic arrangements and help to understand the physical and chemical properties of crystals and how they relate to atomic arrangements.
Full Name: Badreddine Saadali
Status: Associate Lecturer, Class A
Email: badreddine.saadali@univ-oeb.dz
Level: 2nd Year Bachelor's Degree in Geology (TC)
Course Unit: Fundamental Unit F311
Coefficient: 3
Credits: 6
VHH: 4.5 hours
Assessment Methods: Exam 60% - CC 40%
Crystallography is a branch of the exact sciences dedicated to studying the structure of matter at the atomic scale. It involves determining, classifying, and interpreting the geometric structures of solids particularly crystals. This field plays an interdisciplinary role bridging physics, chemistry, molecular biology, materials science, and mineralogy-petrography.

Matter exists in three distinct, geometric states, determined by, the arrangement and mobility of its, constituent molecules: solid, liquid, and gas. In solids, molecules are, tightly packed and fixed in, a structured arrangement. A solid is, characterized by a crystalline, form and/or a periodic, ordered arrangement of atoms.
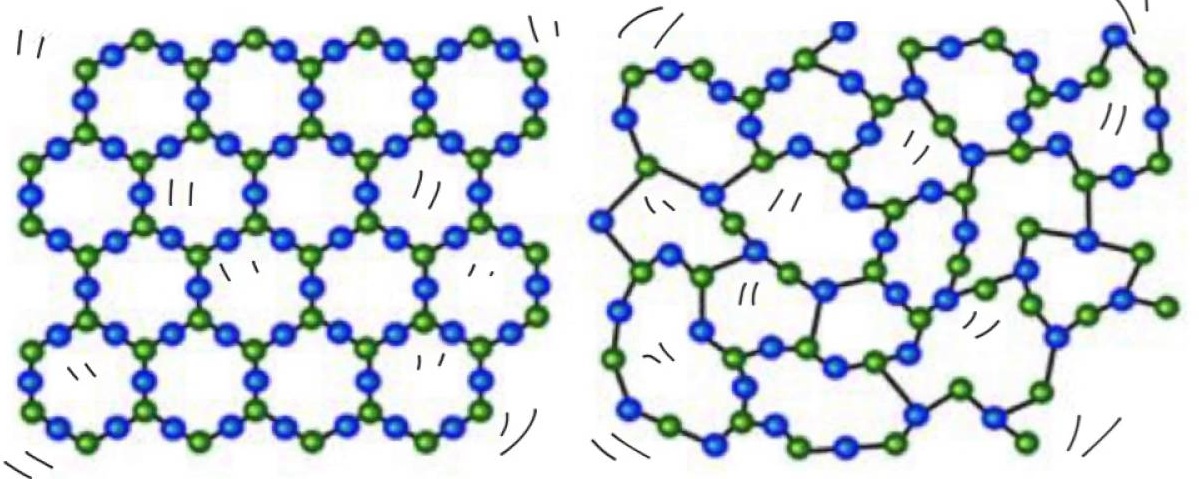
A crystal structure consists of atoms ions or molecules arranged in a specific pattern while a crystal lattice is an infinite mathematical model of points that share the same orientation.
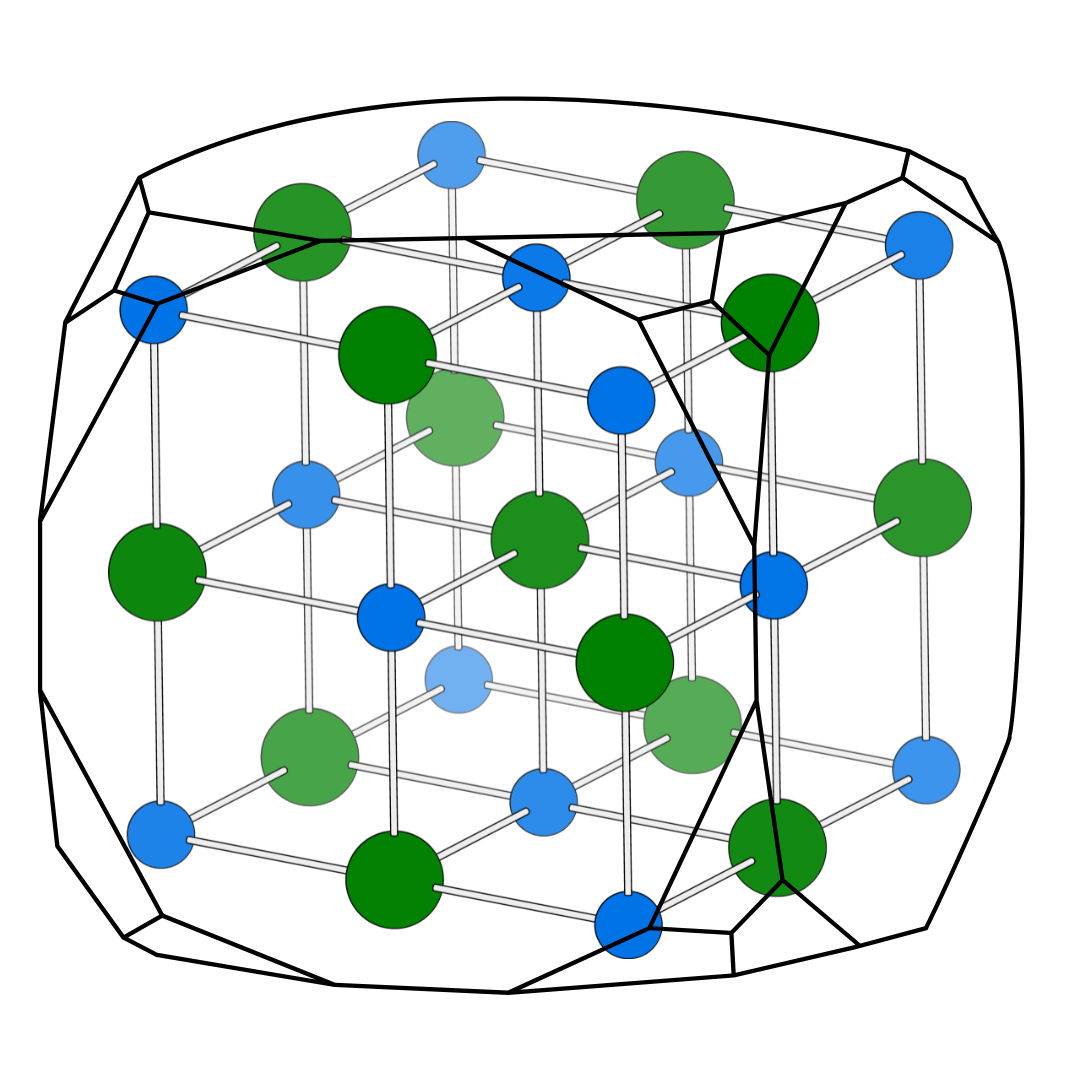
Unit meshes are not always primitive they can also be multiple containing additional nodes beyond those at the corners. These multiple meshes are studied because they reveal the symmetry properties of the crystal.
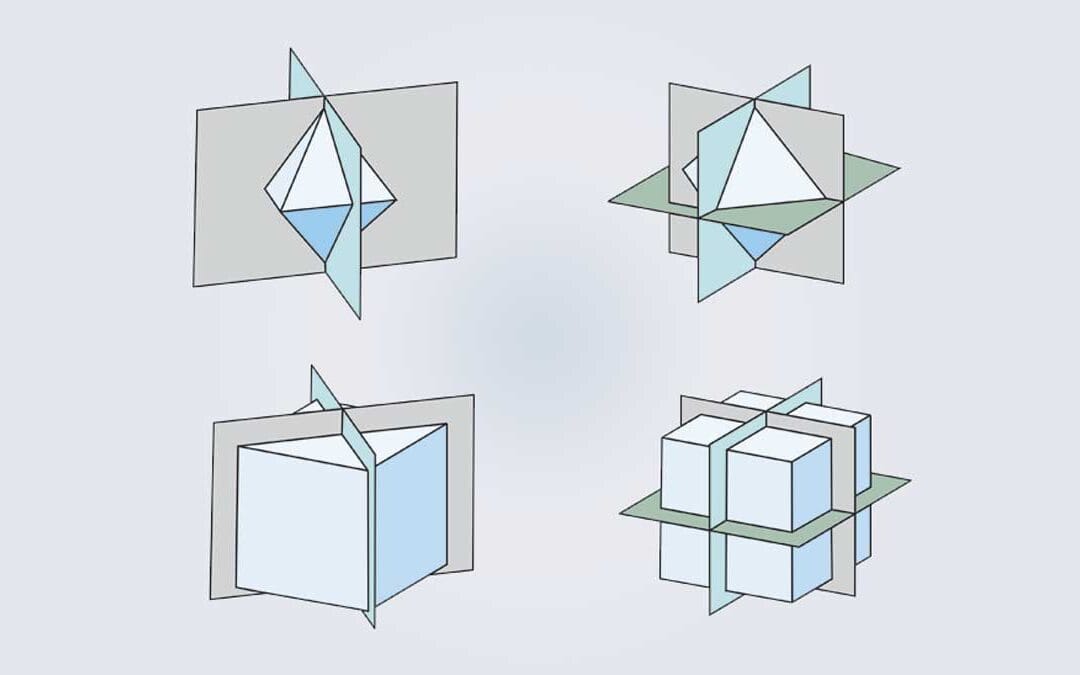
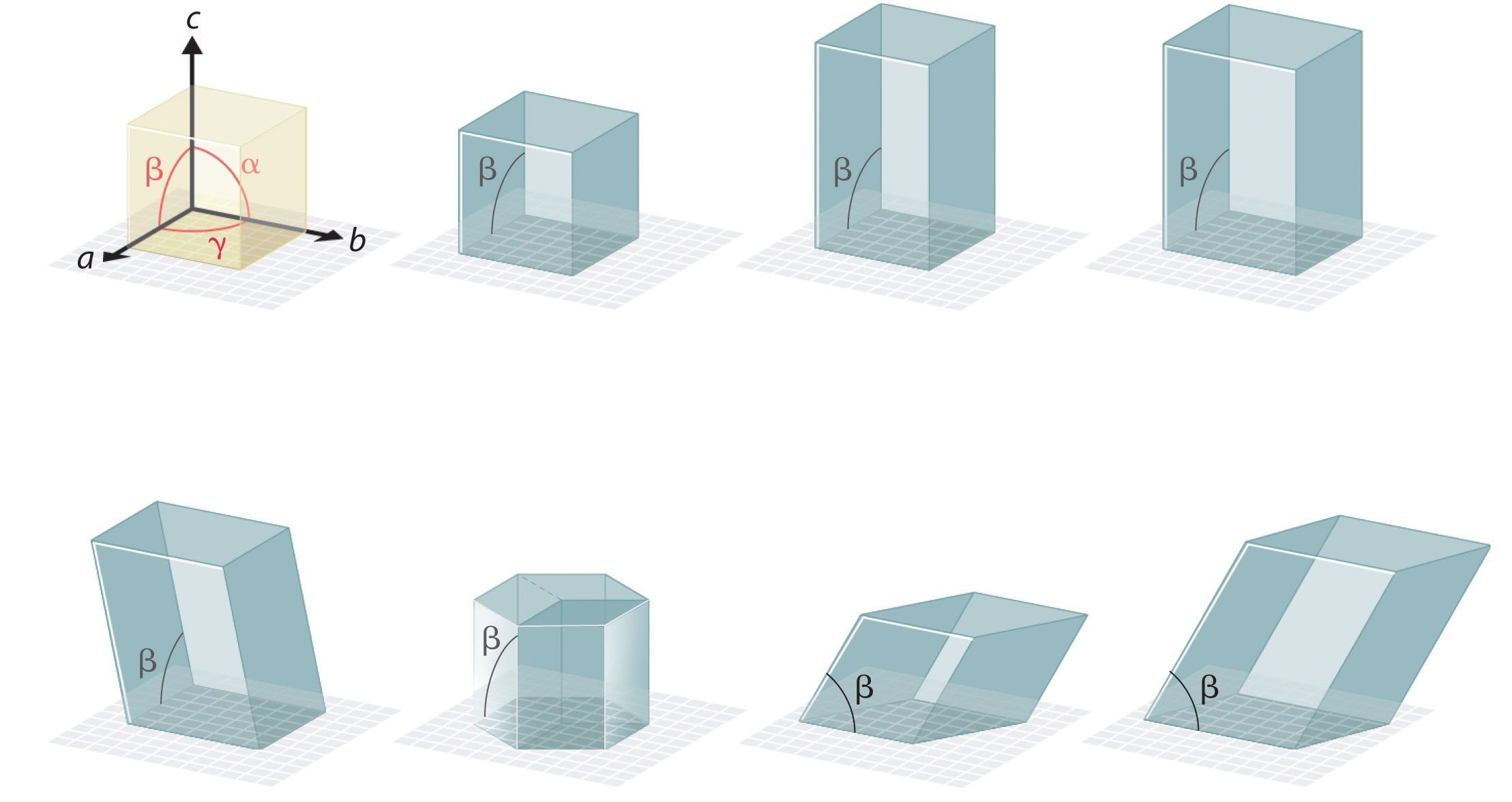
Crystal lattices which form a three-dimensional network are defined by periodicities represented by periodicities (𝑎 ⃗, 𝑏 ⃗ and 𝑐 ⃗). These periodicities correspond to parallelepipeds and there are seven distinct types of crystal lattices each representing the seven major crystal systems. These systems arise from the possible combinations of linear parameters (a, b, and c) and angular parameters (α, β, and γ).
The preservation of a crystal's regular structure is tied to the specific and ordered arrangement of atoms maintained through established interactions that ensure their coherence.
Optics
is the science of, light radiation, a field that has existed for over 2000
years, with the Greeks being the first to explore this concept. The earliest
optical instruments were flat mirrors made from metal, plates or obsidian. The
Romans were able to ignite fire by, focusing sunlight through transparent
glass. The Greek scholar Claudius Ptolemy measured the angles of incidence (i)
and refraction (r) by determining, the deviation of light rays in various,
cases, but he failed to discover the laws governing the refraction of light at
the interface of transparent media.

Akselrod M.S., Bruni F.J. Modern Trends in Crystal Growth and New Applications of Quartz. Journal of Crystal Growth, 2012, 360, 134-145.
Altomare, Angela, et al. Advances in X-ray Analysis: Structure Determination from Powder Diffraction Data. Journal of Applied Crystallography (2009), Vol. 42, pp. 768-775.
Aroyo, Mois I. (ed.). International Tables for Crystallography, Volume A: Space-group Symmetry. Wiley (2016), 920p
Authier A. Early Days of X-ray Crystallography. Oxford University Press, 2013, 480 p
Belfar Abbas Cours et exercices de Cristallographie. Polycopies. Département de Physique Energétique, Faculté de Physique, Université d’Oran, 2014, 79p.
Bloss F.D. Crystallography and Crystal Chemistry: An Introduction. Mineralogical Society of America, 1994, 545 pages.
Chabou Moulley Charaf Cristallographie - Minéralogie. Cours en ligne, 3ème année. Génie Minier, Ecole Polytechnique, Alger, 2011.
Cullity B.D.,Stock S.R. Elements of X-ray Diffraction. 3rd Edition, Prentice Hall, 2001,664 p.
Deferne Jacques Introduction à la cristallographie. Université de Genève, 2015, 110p.
Desiraju, Gautam R., et al. Crystal Engineering: The Design of Organic Solids. Elsevier (1989), 352p.
Dover, Michael, et al. Crystallography in Materials Science: From Structure to Properties. Materials Today (2019), Vol. 22, pp. 1-10.
Férey Gérard Une brève histoire de la cristallographie. Genèse de la cristallographie. L’actualité chimique, 2014, 29-40.
Ferraris, Giovanni, et al. Crystallography of Modular Materials. Oxford University Press (2004), 320p.
Gould, Robert O., et al. Crystallography of Biological Macromolecules: Challenges and Advances. Current Opinion in Structural Biology (2003), Vol. 13, pp. 1-8.
Helliwell, John R. Macromolecular Crystallography with Synchrotron Radiation. Cambridge University Press (1992), 480p.
International Union of Crystallography (IUCr). International Tables for Crystallography. 6th Edition, Wiley, 2019, 1200 pages.
Jana, S.C. (et al.). Crystallography and Crystal Defects. 3rd Edition. Wiley. 2020, 480p.
Kocks, U.F. (et al.). Texture and Anisotropy: Preferred Orientations in Polycrystals and Their Effect on Materials Properties. Cambridge University Press. 1998, 676p.
Krivovichev, S.V.. Structural Crystallography of Inorganic Oxysalts. Oxford University Press. 2009, 320p.
Lecomte Claude Cours de cristallographie. Cristallographie, Résonance Magnétique et Modélisations. Université de Lorraine, 2013, 140p.
Massa Werner Crystal Structure Determination. 2nd edition. Springer-Verlag Berlin and Heidelberg GmbH & Co. K, 2004, 212p.
Nardelli, M. (et al.). Crystallography of Modulated Structures. 1st Edition. Springer. 1986, 200p.
Nielsen, R.H. (et al.). Crystallography and Crystal Chemistry of Materials. 1st Edition. Springer. 1990, 300p
Pecharsky V.K., Zavalij P.Y. Fundamentals of Powder Diffraction and Structural Characterization of Materials. Springer, 2009, 748 pages.
Rogers, D.. Crystallography: An Introduction for Earth Science (and Other Solid State) Students. 1st Edition. Pergamon Press. 1975, 200p
Schwarzenbach Dieter Crystallography. 1st edition. PPUR presses polytechniques, 1993, 237p.
Thalal Abdelmalek La symétrie en cristallographie. International Year of Crystallography, 2014, 72p.
Von Laue M. Concerning the Detection of X-ray Interference Phenomena. Annalen der Physik, 1912, 41(10), 971-988.
Wyrouboff Grégoire Manuel pratique de cristallographie. 1ère édition. Gauthier-Villars et fils, Imprimeurs-Libraires, Paris, 1889, 344p.
Yahi H. Polycopié de Cours Cristallographie. Univ Guelma. 2017, 69p.
Zuppiroli Libero, Bussac Marie-Noëlle Traité de la lumière. PPUR presses polytechniques, 2009, 481p.